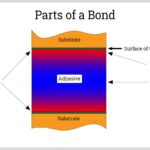
Glue is a popular adhesive used in a variety of applications. It is made up of a combination of polymers and resins, which create an adhesive that is stronger than the individual components. On a chemical level, the glue works by bonding two surfaces together, with the adhesive acting as a bridge between them. The polymer molecules are attracted to both the surfaces, creating a strong bond. The resin then reinforces this bond, making it even stronger.
When the glue is applied to the surfaces, the molecules from the adhesive spread out, forming a thin film between the two surfaces. This film is then chemically and physically attracted to the surfaces, creating a strong bond. The molecules then form a strong bond that cannot be broken easily. This bond can last for years, depending on the type of glue and the environment it is in. Glues are available in different grades for different applications, making it a versatile adhesive. Overall, glue works by forming a strong bond between two surfaces through the combination of polymers and resins. The molecules spread out, creating a thin film that is then attracted to the surfaces and creates a strong bond that can last for years.
What is the chemical formula of glue
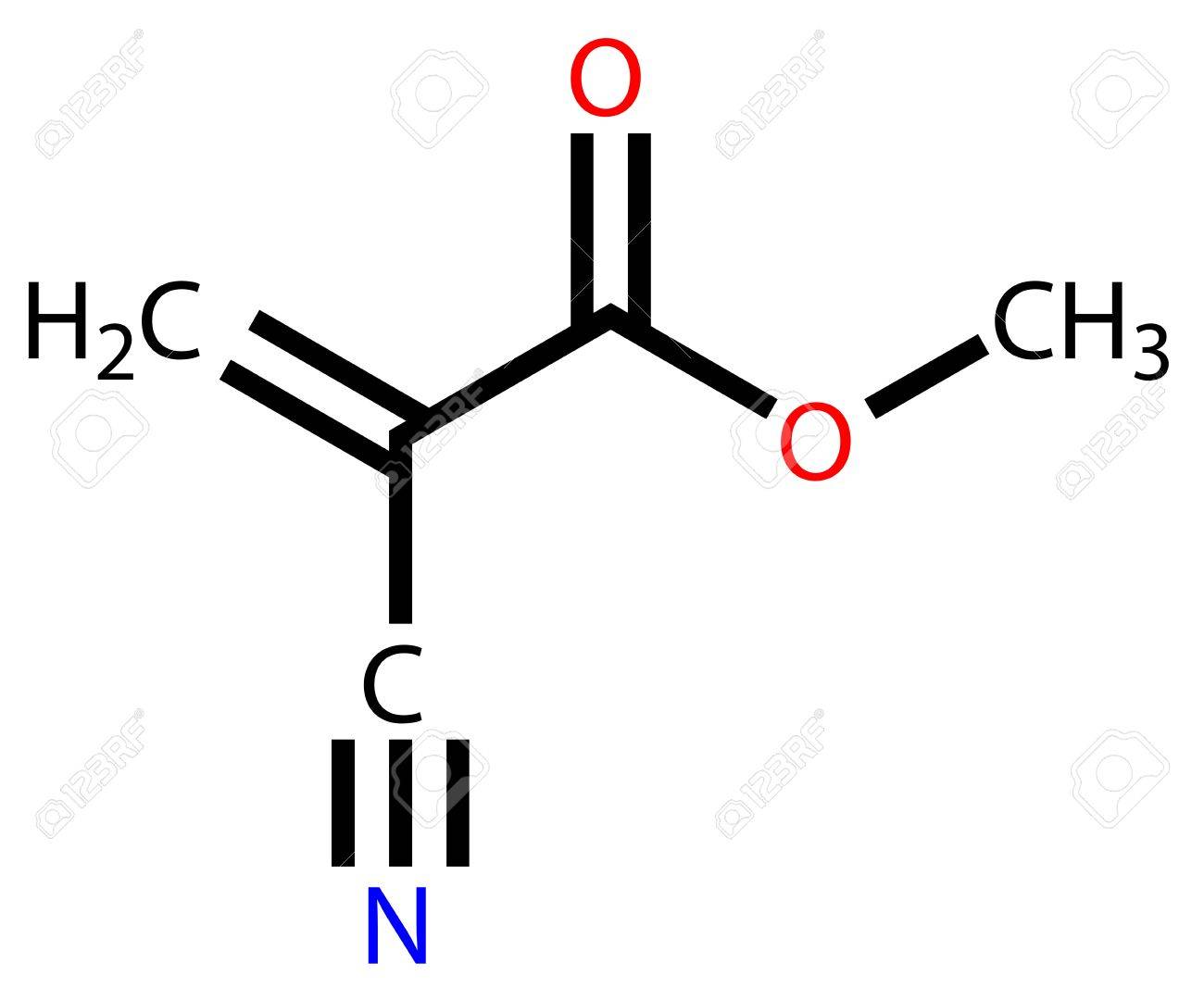
Glue molecules are usually bonded together in a matrix, which gives the glue its strength and flexibility. The chemical formula of glue depends on the type of glue that is used. For example, the most common type of glue, polyvinyl acetate (PVA) glue, has the chemical formula C4H6O2. PVA glue is composed of vinyl acetate monomers, which are molecules that contain the chemical elements carbon, hydrogen, and oxygen. These monomers form links between one another, forming a chain that creates a durable bond.
Another type of glue, cyanoacrylate, has the chemical formula C7H5NO2. This type of glue is composed of molecules that contain the chemical elements carbon, hydrogen, nitrogen, and oxygen. The molecules in cyanoacrylate glue form strong links between one another due to the presence of a molecule known as an ethyl cyanoacrylate, which has a high affinity for sticking to other materials. Overall, the chemical formula of glue depends on the type of glue being used. The most common types of glue are PVA and cyanoacrylate. PVA glue is composed of vinyl acetate monomers while cyanoacrylate glue is composed of molecules with the chemical formula C7H5NO2. Both types of glue work by forming strong links between one another, which creates a durable bond.
What chemical elements make up glue?
On a chemical level, glue is composed of a variety of elements, including oxygen, carbon, hydrogen, and nitrogen. These elements form chemical bonds between different molecules, which helps the glue stick to other objects. Glue also typically contains resins and polymers, which are composed of long chains of carbon atoms. These carbon atoms are highly reactive and form strong bonds with other elements, such as oxygen and hydrogen. These strong bonds give glue its adhesive properties, allowing it to stick to things.
The chemical properties of glue also depend on the type of glue being used. For example, an epoxy glue contains a mixture of epoxy resin and curing agent, which form a hard, durable bond when mixed together. On the other hand, an animal-based glue, such as hide glue, contains proteins that can form strong bonds when heated. Finally, some glues are made up of polymers that contain a special chemical called an emulsifier. This emulsifier helps to break down the glue into smaller particles, which helps it adhere to surfaces better. In conclusion, glue is a complex substance that is composed of a variety of chemical elements, including oxygen, carbon, hydrogen, and nitrogen. These elements and other ingredients, such as resins and polymers, form strong chemical bonds that give glue its adhesive properties.
What are chemicals in glue?
Generally, the chemicals used in glue are polyvinyl acetate and polyvinyl alcohol. Together, these create a strong bond when applied to the surface of two materials. Polyvinyl acetate is a polymer created when an acid reacts with an alcohol. When polyvinyl acetate is added to water, it forms into a sticky glue. This glue is then used to stick two surfaces together.
Polyvinyl alcohol is also a polymer created when an alcohol reacts with an aldehyde. This polymer helps to prevent the glue from dissolving when it is applied to materials such as paper, cloth, and wood. The mixture of polyvinyl acetate and polyvinyl alcohol creates a strong bond between two surfaces when it is applied. This bond is created by the polymers and the water in the glue. The polymers help to hold the two surfaces together, while the water helps to soften and expand the glue, making it easier to apply. The chemicals in glue are essential for creating strong, durable bonds between materials. Without the polymers and water, the glue would not be able to form a strong bond between two surfaces. Thus, glue would not be able to work effectively.
What are the rules in making chemical formula?
Glue is made of polymers, which are long chain molecules composed of repeating units of the same type. In chemistry, chemical formulas are used to represent the components of a molecule. The rules for chemical formulas are used to communicate the components in a molecule without having to write out the full name. The basic rule for writing chemical formulas is to write the chemical symbol for each element present in the molecule. The chemical symbol is made up of one or two letters from the periodic table.
For example, the symbol for hydrogen is H and the symbol for oxygen is O. When two or more elements combine to form a compound, the chemical formula must indicate the number of atoms of each element that are present. For example, in the molecule water (H2O), there are two atoms of hydrogen and one atom of oxygen. In the case of polymers, the chemical formula for the repeating unit must also be included. For example, the common polymer polyethylene is represented as (C2H4)n, where n is the number of repeating units. Finally, when writing a chemical formula for a glue, the ingredients must be accounted for. Glue is typically composed of a polymer, additives, and a solvent. The chemical formula must include the symbols for each element and the number of atoms for each element in each component.
What is the chemistry of glue?
On a chemical level, glue is made up of long chains of molecules that are flexible and have an adhesive property. When glue is applied to a surface, the solvent in the glue starts to evaporate. This makes the glue viscous and sticky, allowing the glue molecules to adhere to the surface. As the solvent evaporates, the molecules in the glue form strong intermolecular bonds with the molecules on the surface. This creates a strong bond between the two surfaces, which is what makes glue so effective.
The strength of the bond will depend on the type of glue used and the surface it is applied to. Different types of glues have different polymer compositions, which can provide more or less adhesion depending on the type of surface. For example, some glues are better for use on porous surfaces such as wood, while other glues are better for use on non-porous surfaces such as metal. When it comes to removing glue, it is important to remember that different types of glue require different methods. Heat, chemical solvents, and mechanical force are all options for breaking the bonds between the surfaces and the glue. Overall, glue is an important adhesive that is used in many different applications. Its chemical composition allows it to form strong bonds with a variety of surfaces, making it an invaluable tool in many industries.